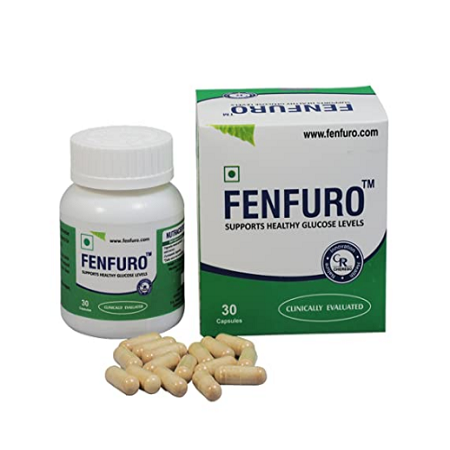
Helps to maintain healthy blood glucose levels through Herbs.
|
|
Clinical Evaluation of Fenugreek Seed Extract in 2 58 Patients with Type- 2 Diabetes : An Add-On Study at Two Medical Colleges in India.
Efficacy Conclusions
- A significant decrease in HbA1c levels was observed as compared to respective baseline value and as compared to placebo after weeks.
- Fenfuro caused significant reduction in the fasting blood sugar levels.
- Fenfuro caused significant reduction in the post prandial (PP) blood sugar levels as compared to Placebo group as well as compared to respective baseline value.
- 83% of the patients reported decrease in fasting sugar levels in Fenfuro treated group as compared to 62% in Placebo treated group.
- 89% of the patients reported decrease in PP sugar levels in Fenfuro treated group as compared to 72% of the patients in the Placebo treated group.
- 48.8% of the patients reported reduced dosage of anti diabetic therapy in Fenfuro treated group, where as 18.05% of the patients reported reduced dosage of anti diabetic therapy in Placebo treated group.
- Fasting glucose: The mean fasting glucose levels were significantly decreased in FENFURO-treated patients, whereas these levels increased in on-going anti-diabetic therapy-treated patients. FENFURO caused 38.26% decrease in fasting glucose levels on completion of the treatment. Such decrease in fasting glucose levels was observed in 95.2% of the study population on completion of the treatment with FENFURO.
- Post-prandial (PP) glucose: FENFURO caused significant decrease in PP glucose levels on completion of the treatment as compared to the on-going anti-diabetic therapy-treated population. The decrease in mean PP glucose levels were up to 44.04% in the FENFURO-treated study population on completion of the treatment. As observed on completion of the treatment with FENFURO, 88.10% of study population shown to have decrease in PP glucose levels.
- Glycated hemoglobin (HbA1c): FENFURO treatment resulted in normalizing the mean HbA1c levels of the study population. The HbA1c levels decreased significantly in the study population of both groups on completion of the treatment. Mean HbA1c levels decreased up to 34.70% in
- FENFURO-treated group whereas on-going anti- diabetic therapy caused 21.51% decrease in HbA1c levels. These HbA1c levels came to normal range (Good control range – 4.5-6.3%) in FENFURO-treated study population whereas they were still abnormal (Poor control levels – 7.6%) in on-going anti-diabetic therapy treated population till 12 weeks of treatment.
Safety Conclusions
- No significant change in serum SGOT, SGPT & ALP activities was observed, indicating investigational product (Fenfuro-fenugreek seed extract) was safe for liver functioning.
- No significant change in blood urea nitrogen and creatinine levels was observed indicating investigational product (Fenfuro-fenugreek seed extract) was safe for kidney functioning.
- No significant change in hematological parameters was observed.
- Keeping hematological and biochemical results in view, investigational product was safe for consumption.
- No significant change in the liver function tests (serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase, alkaline phosphates activities and serum bilirubin levels) was observed on completion of the treatment.
- No significant change in the serum urea levels and creatinine levels was observed on completion of the treatment.
- No significant change in the hematological parameters was observed on completion of the treatment.
Unique features of FENFUROTM
- FENFUROTM is a first of its kind product derived from single herb with proven results for maintaining blood sugar levels.
- Scientific Research supports the non-toxicity of the product.
- Scientifically processed without affecting the chemical properties of the active ingredient to give maximum benefit.

*Complete clinical data available on request

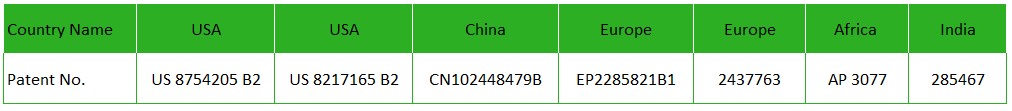
Innovative product FENFURO is now protected with following patents :






Reviews
There are no reviews yet.